Premium Wrist Splint for Sale - Max Support & Pain Relief
Advancing Orthopedic Care: A Comprehensive Guide to High-Performance Wrist and Thumb Splints
In the demanding landscape of modern medical care and occupational health, the efficacy of rehabilitative and supportive devices is paramount. This detailed exposition serves as an authoritative resource for B2B professionals seeking superior wrist splint for sale solutions. We delve into the critical aspects of design, manufacturing, and application, emphasizing the rigorous standards that define top-tier orthopedic support. From acute injury management to long-term chronic condition support, the right splint offers indispensable immobilization, pain relief, and accelerated recovery. This article will explore the intricate technical specifications, diverse application scenarios, and compelling advantages that distinguish leading products in the market, including specialized variants such as the finger splint with wrist support, flexible thumb splint, and the robust metal thumb brace.
Industry Trends in Orthopedic Support Devices
The orthopedic support market is experiencing dynamic growth, driven by an aging global population, increased awareness of sports injuries, and advancements in material science. Key trends influencing the development and procurement of devices like the left hand brace with thumb support include:
- Personalization and Customization: Growing demand for patient-specific solutions, often facilitated by 3D printing and advanced molding techniques, to enhance fit and therapeutic outcomes.
- Lightweight and Breathable Materials: A shift towards advanced composites, perforated fabrics, and moisture-wicking materials that offer superior comfort for extended wear without compromising support.
- Smart Integration: Emerging technologies incorporating sensors for monitoring patient compliance, movement, and healing progress, providing valuable data for clinicians.
- Evidence-Based Design: Increased emphasis on clinical trials and scientific validation to prove the efficacy of splint designs, aligning with stricter regulatory requirements.
- Sustainability: A rising focus on eco-friendly materials and manufacturing processes, appealing to environmentally conscious healthcare providers.
These trends collectively underscore a market moving towards more efficient, comfortable, and therapeutically advanced solutions, setting higher benchmarks for any wrist splint for sale.
Precision Manufacturing Process of Orthopedic Splints
The production of a high-quality wrist splint for sale involves a meticulously controlled manufacturing process, ensuring both therapeutic efficacy and patient safety. Our commitment to excellence is reflected in every stage, from material selection to final quality assurance.
Process Flow Schematic:
1. Material Selection & Sourcing
Utilizing medical-grade thermoplastics (e.g., polyethylene, polypropylene), aluminum alloys for rigid supports, breathable fabrics (e.g., neoprene, nylon/spandex blends) for comfort, and hook-and-loop fasteners. All materials comply with biocompatibility standards (ISO 10993).
2. Design & Prototyping
CAD software is used for precise anatomical contouring. Prototypes undergo ergonomic testing and biomechanical analysis to optimize immobilization and comfort. This phase ensures the design aligns with therapeutic objectives, especially for products like the injured thumb protector.
3. Component Manufacturing
Injection Molding: For plastic shells and buckles. CNC Machining: For precision shaping of metal components (e.g., aluminum stays for a metal thumb brace). Textile Cutting & Stitching: Automated and manual processes for fabric components, ensuring consistent sizing and seam integrity.
4. Assembly & Finishing
Manual and semi-automated assembly integrates all components. This includes securing rigid stays within fabric pockets, attaching straps, and applying padding. Surface treatments may be applied to metal components for corrosion resistance.
5. Quality Control & Testing
Each batch undergoes rigorous testing: material strength, dimensional accuracy, strap durability, fastener integrity, and functional performance. Adherence to ISO 13485 (Medical Devices Quality Management) and FDA regulations is paramount. Products are tested for a projected service life of 6-12 months under typical usage.
6. Packaging & Sterilization (if applicable)
Products are packaged in sterile or clean environments, often with clear instructions for use. Traceability is maintained through batch numbering and labeling, ensuring compliance and patient safety.
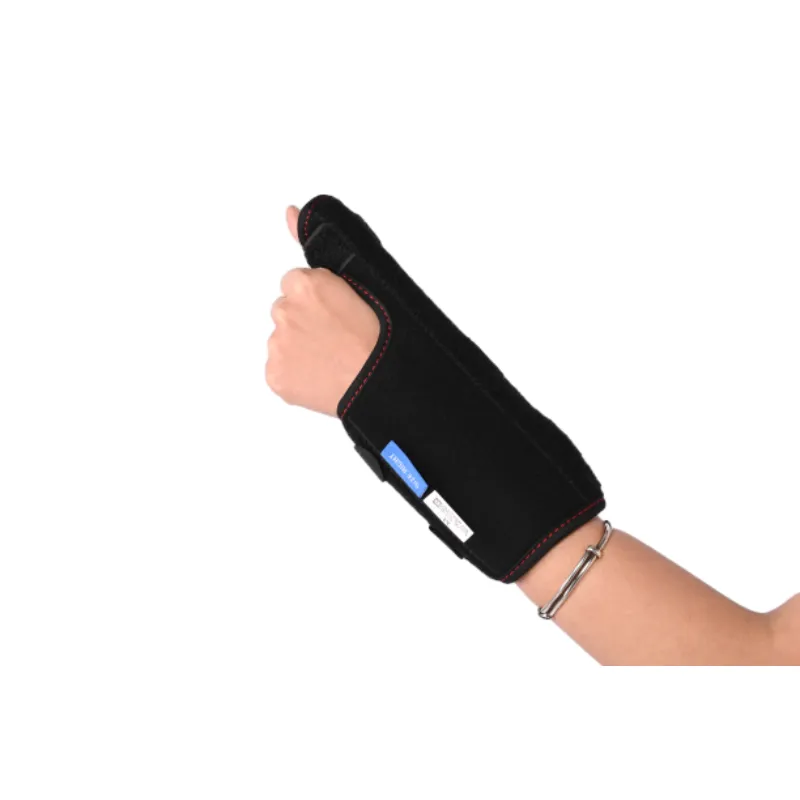
Figure 1: Advanced manufacturing techniques ensure precision and durability in orthopedic splints.
Target Industries and Advantages:
Our orthopedic support devices, including those designed as a wrist splint for sale, are indispensable across a range of sectors that demand reliable patient care solutions:
- Healthcare Facilities: Hospitals, clinics, and rehabilitation centers for post-operative care, fracture management, and chronic conditions. Advantages include clinical efficacy and robust construction for repeated use.
- Sports Medicine: Athletic training centers and sports clinics for managing sprains, strains, and overuse injuries. Benefits include dynamic support and expedited return to activity.
- Occupational Therapy: Workplaces requiring injury prevention or ergonomic support. Products offer comfort and functional support during work tasks, preventing aggravation of conditions like Carpal Tunnel Syndrome.
- Home Healthcare: For long-term patient support and rehabilitation. Our splints offer ease of application and wearer comfort, promoting patient compliance.
In typical application scenarios, these advanced manufacturing processes translate directly into tangible advantages such as superior patient comfort, enhanced therapeutic outcomes through precise immobilization, and exceptional product longevity, contributing to energy saving in the long run by reducing the need for frequent replacements. The material selection also ensures corrosion resistance and maintains structural integrity even in varied environmental conditions within clinical settings.
Technical Specifications and Performance Parameters
Understanding the technical specifications of a wrist splint for sale is crucial for procurement specialists and medical professionals. Our products are engineered to deliver consistent performance, backed by stringent quality control.
Product Specification Table: Thumb Splint with Wrist Support (Representative Model)
| Parameter | Specification |
|---|---|
| Product Type | Thumb Splint with Wrist Support (e.g., De Quervain's Splint) |
| Primary Materials | Medical-grade Neoprene/Nylon, Lightweight Aluminum Stay, Hook-and-Loop Fasteners |
| Support Structure | Anatomically pre-shaped removable aluminum stay for thumb and radial wrist |
| Immobilization Level | Moderate to Rigid for thumb CMC and MP joints, and wrist |
| Available Sizes | Small, Medium, Large, X-Large (Unisex) |
| Hand Compatibility | Left or Right Hand specific; some models ambidextrous |
| Breathability | High (perforated neoprene or mesh panels) |
| Weight | Approx. 80-150 grams (depending on size) |
| Certifications | ISO 13485:2016, CE Marked, FDA Registered |
| Service Life | Recommended 6-12 months under normal use conditions |
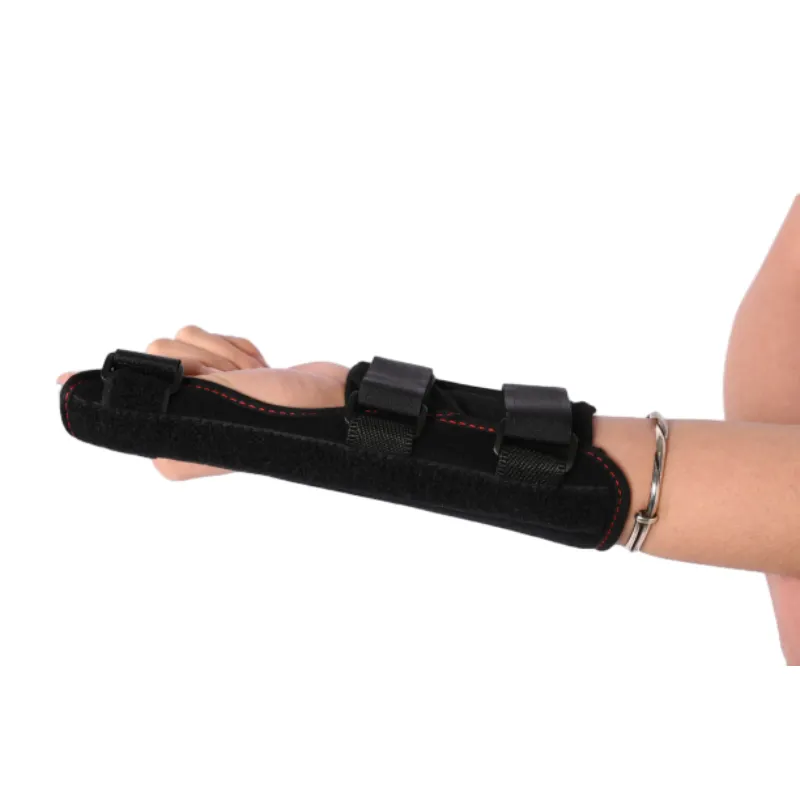
Figure 2: Ergonomic design combined with robust materials ensures optimal therapeutic support.
Our continuous product development ensures that each flexible thumb splint and comprehensive wrist support integrates the latest advancements in materials science and biomechanical engineering. This commitment guarantees superior performance in terms of patient comfort, functional stability, and durability.
Diverse Application Scenarios
The versatility of a well-designed wrist splint for sale extends to a multitude of clinical and rehabilitative contexts. These devices are fundamental for managing a spectrum of upper extremity conditions, providing targeted support and aiding recovery.
- Carpal Tunnel Syndrome (CTS): Night and daytime wear to maintain the wrist in a neutral position, alleviating pressure on the median nerve.
- De Quervain's Tenosynovitis: Specific thumb and wrist immobilization provided by a finger splint with wrist support or a specialized thumb splint to reduce inflammation of the thumb tendons.
- Post-Fracture Management: Supporting the wrist and/or thumb after plaster cast removal, ensuring gradual mobilization and protection during the healing phase.
- Sprains and Strains: Providing stable support to injured ligaments and tendons in the wrist and thumb, promoting healing and preventing re-injury.
- Arthritis (Rheumatoid and Osteoarthritis): Offering pain relief and joint stability, particularly beneficial for the thumb CMC joint, which often benefits from an injured thumb protector.
- Post-Surgical Recovery: Critical for protecting surgical sites and ensuring proper alignment during the initial recovery period following procedures on the wrist or thumb.
- Repetitive Strain Injuries (RSI): Prophylactic use in occupational settings to prevent the onset or exacerbation of conditions due to repetitive hand and wrist movements.
The adaptability of our range, including options like the left hand brace with thumb support and the robust metal thumb brace, ensures that healthcare providers can select the precise level of immobilization and support required for each unique patient presentation.
Technical Advantages and User Experience
Our range of wrist and thumb splints represents the zenith of orthopedic engineering, offering distinct technical advantages that translate into superior patient outcomes and operational efficiency for healthcare providers.
- Anatomically Contoured Design: Precision molding and stitching ensure an optimal fit that follows the natural curves of the wrist and hand, providing effective, localized compression and stabilization without creating pressure points. This design minimizes soft tissue irritation and enhances comfort during extended wear.
- Advanced Material Composition: Utilization of high-grade, breathable, moisture-wicking fabrics (e.g., neoprene blends with perforations) to prevent skin maceration and maintain patient hygiene. The integration of lightweight yet rigid aluminum or thermoplastic stays ensures robust immobilization while reducing bulk.
- Adjustable Compression Systems: Features like multiple hook-and-loop straps and elastic closures allow for variable compression and a customizable fit, accommodating swelling variations and ensuring consistent therapeutic pressure. This adaptability is key for a truly versatile wrist splint for sale.
- Removable and Malleable Stays: Many models include removable or heat-moldable stays, allowing clinicians to fine-tune the degree of immobilization or progressive mobilization as rehabilitation advances. This feature is particularly valuable in products like the flexible thumb splint.
- Hypoallergenic and Latex-Free: All materials are carefully selected to be hypoallergenic and latex-free, minimizing the risk of allergic reactions and making them suitable for a broader patient demographic.
- Durability and Ease of Maintenance: Constructed for longevity, our splints are designed to withstand daily wear and tear and are easy to clean, ensuring a hygienic and cost-effective solution for both patients and healthcare facilities.
These technical advantages collectively contribute to enhanced patient compliance, faster recovery times, and a reduction in associated healthcare costs, making our products a strategic investment for medical institutions.
Vendor Comparison: Selecting the Optimal Orthopedic Partner
When evaluating a wrist splint for sale, B2B purchasers must consider more than just the product itself. The choice of vendor significantly impacts product quality, supply chain reliability, and long-term partnership value.
Orthopedic Solutions Vendor Comparison Table (General Categories):
| Criterion | Specialized Orthopedic Manufacturer (e.g., JHO Orthopedic) | General Medical Supplier/Distributor |
|---|---|---|
| Product Focus & Depth | Deep specialization in orthopedic devices; extensive range of specific splint types (e.g., dedicated finger splint with wrist support, injured thumb protector). | Broad catalog across many medical categories; limited depth in specific orthopedic niches. |
| Quality Control & Certifications | In-house R&D, manufacturing, and rigorous testing to ISO 13485, FDA, CE standards. Direct control over material sourcing and process. | Relies on manufacturer's certifications; less direct control over production quality. |
| Customization & OEM Capabilities | High capacity for custom solutions, bulk OEM orders, private labeling, and design modifications for products like the left hand brace with thumb support. | Limited to existing product lines; customization usually requires direct manufacturer engagement. |
| Technical Support & Expertise | Direct access to product engineers and clinical specialists. In-depth knowledge for troubleshooting and application guidance. | General sales support; may refer to manufacturer for complex technical inquiries. |
| Innovation & R&D | Ongoing investment in new materials, designs, and therapeutic advancements. Proactive in addressing market needs. | Primarily focuses on distribution; less direct involvement in product innovation. |
| Pricing Structure | Often more competitive for bulk orders due to direct manufacturing; potential for better long-term value. | Pricing includes distribution margins; may be higher for specialized or custom orders. |
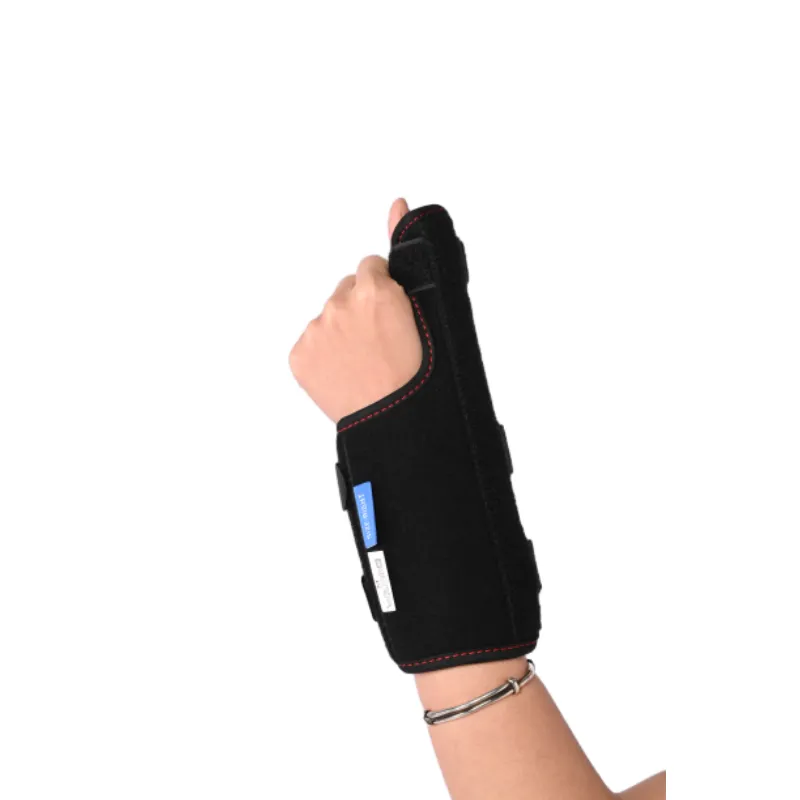
Figure 3: A diverse range of orthopedic supports caters to varied clinical requirements.
Partnering with a specialized manufacturer like JHO Orthopedic ensures direct access to expertise, quality assurance, and the flexibility to meet evolving clinical demands, ultimately providing superior value for your investment in a wrist splint for sale and related orthopedic products.
Customized Solutions & OEM Partnership Opportunities
Understanding that off-the-shelf solutions may not always meet every unique requirement, we offer comprehensive customized solutions and robust OEM (Original Equipment Manufacturer) partnership programs for high-volume purchasers of wrist splint for sale products.
- Material Adaptation: Custom requests for alternative fabric types (e.g., enhanced moisture-wicking, antimicrobial treatments, or specific compression ratios) or different metal alloys for reinforced stays.
- Design Modifications: Adjustments to splint geometry, strap configurations, padding thickness, or closure mechanisms to suit specific patient populations or clinical protocols. This can include specialized designs for a flexible thumb splint.
- Sizing and Fit Optimization: Development of bespoke sizing charts or models for atypical anthropometric data, ensuring optimal fit for diverse demographic needs.
- Branding and Packaging: Private labeling, custom branding, and specialized packaging solutions to align with your institutional identity or specific distribution channels.
- Integration of Smart Features: Exploration of incorporating embedded sensors or monitoring capabilities for advanced data collection in rehabilitative settings.
Our R&D team works closely with partners to transform conceptual requirements into fully compliant, high-performance orthopedic solutions, maintaining strict adherence to medical device standards (ISO 13485, FDA). This collaborative approach ensures that whether you require a bespoke metal thumb brace or a specialized left hand brace with thumb support, our manufacturing capabilities are perfectly aligned with your strategic objectives.
Application Case Studies: Demonstrating Real-World Impact
Our commitment to quality and efficacy is best illustrated through successful real-world applications. These case studies highlight how our specialized wrist splint for sale products deliver tangible benefits to healthcare systems and patients.
Case Study 1: Large Hospital Network Procurement
A national hospital network sought a unified source for high-quality orthopedic supports, including thumb splints and wrist braces, to standardize patient care across its numerous facilities. They required products that were durable, patient-friendly, and compliant with the highest medical standards.
Solution Provided: We partnered with the network to supply a comprehensive range of our ISO 13485 certified splints, including various sizes of the finger splint with wrist support and specific injured thumb protector models. We implemented a streamlined logistics system ensuring consistent inventory and timely delivery to all hospital locations.
Results: The network reported a 15% reduction in re-injury rates due to superior splint efficacy, a 20% increase in patient satisfaction scores attributed to enhanced comfort and fit, and significant cost savings through competitive bulk pricing and reduced administrative overhead in procurement.
Case Study 2: Sports Rehabilitation Clinic Enhancements
A prominent sports rehabilitation clinic specializing in professional athletes required robust and dynamic wrist and thumb supports that could withstand intense activity and provide reliable progressive rehabilitation. Their existing suppliers offered products that lacked either durability or the necessary flexibility for varying stages of recovery.
Solution Provided: We supplied them with our advanced range, including a specially developed flexible thumb splint and custom-fitted metal thumb brace options. These products featured removable and heat-moldable stays, allowing for precise adjustment of immobilization as athletes progressed through recovery phases.
Results: The clinic observed a 10% faster return-to-play rate for athletes with hand and wrist injuries, attributed to the optimal balance of support and mobility provided by our splints. Clinicians praised the durability and adaptability, leading to improved patient outcomes and a stronger reputation for the clinic.
Frequently Asked Questions (FAQ)
Q: What certifications do your wrist splint for sale products hold?
A: Our products are manufactured under strict quality management systems and are typically certified to ISO 13485:2016 (Medical Devices Quality Management). Many models also hold CE Marking for European conformity and are FDA Registered for the United States market, ensuring global compliance and safety.
Q: What is the estimated lead time for bulk orders of a left hand brace with thumb support?
A: Standard bulk orders typically have a lead time of 4-6 weeks from order confirmation, depending on customization requirements and order volume. Expedited options may be available upon request. We maintain efficient supply chains to minimize delays.
Q: What warranty do you offer on your orthopedic splints?
A: We offer a standard 12-month warranty on all manufacturing defects from the date of purchase. This warranty covers material flaws and workmanship under normal use conditions. Specific terms and conditions are available upon request.
Q: Can we request custom branding or private labeling for a metal thumb brace?
A: Yes, we specialize in OEM services and can accommodate custom branding, private labeling, and specific packaging requirements for bulk orders. Our design team will work with you to integrate your brand identity seamlessly.
Q: What kind of customer support is available post-purchase?
A: Our dedicated customer support team is available to assist with product inquiries, technical support, order tracking, and warranty claims. We provide comprehensive after-sales service to ensure complete satisfaction and uninterrupted operational efficiency for our partners.
Conclusion
The selection of a reliable wrist splint for sale is a critical decision for any healthcare provider or medical supply chain. By prioritizing products manufactured with precision, utilizing advanced materials, and adhering to rigorous international standards, institutions can ensure superior patient outcomes, enhanced operational efficiency, and long-term cost-effectiveness. Our commitment to innovation, quality, and comprehensive support positions us as an ideal partner for your orthopedic device needs. We invite you to explore our full range of solutions and experience the difference that dedicated expertise makes in patient care.
Authoritative References:
- International Organization for Standardization (ISO). ISO 13485:2016 Medical devices — Quality management systems — Requirements for regulatory purposes.
- U.S. Food and Drug Administration (FDA). Medical Devices – Premarket Notification [510(k)].
- American Academy of Orthopaedic Surgeons (AAOS). Clinical Practice Guidelines for Carpal Tunnel Syndrome.
- British Journal of Sports Medicine. Evidence-based rehabilitation protocols for common wrist and hand injuries.
- Journal of Hand Surgery. Biomechanical analysis of thumb splints for basilar joint arthritis.
This is the first article
-
Optimal Back & Belly Support During Pregnancy | Pain Relief & ComfortNews Aug.27,2025
-
Black Arm Sling for Wrist Injury | Shoulder Support & ComfortNews Aug.26,2025
-
Hard Cervical Collar: Firm Support, Chin Rest & Pain ReliefNews Aug.21,2025
-
Abduction Pillow Brace: Optimal Shoulder Support & RecoveryNews Aug.19,2025
-
Butterfly Brace for Broken Collar Bone Support & HealingNews Aug.18,2025





















