Shoulder Pain Belt Price: Best Compression Sleeve & Brace
Navigating the Orthopedic Device Market: Insights into Shoulder Support Solutions and Pricing Trends
In the dynamic landscape of orthopedic rehabilitation, the demand for effective, comfortable, and durable shoulder support solutions continues to grow. As healthcare providers, rehabilitation specialists, and patients seek optimal outcomes for a range of shoulder pathologies, understanding the nuanced aspects of devices like the Shoulder Abduction Orthosis becomes paramount. This comprehensive analysis delves into the critical factors influencing the market, from advanced material science and precision manufacturing to clinical efficacy and economic considerations. We address key inquiries surrounding device selection, specifically focusing on the shoulder pain belt price, alongside a detailed exploration of related solutions such as compression sleeves for rotator cuff injury, rotator cuff compression braces, shoulder compression sleeves for lifting, shoulder compression sleeve wraps, and shoulder compression straps. The global market for orthopedic braces and supports, valued at approximately USD 5.2 billion in 2023, is projected to reach USD 7.8 billion by 2030, driven by an aging population, rising sports injuries, and increased awareness of non-invasive treatment options. Within this segment, shoulder-specific devices represent a significant and growing share, with advancements in material technology, biomechanical design, and patient-specific customization driving innovation. Our objective is to provide B2B decision-makers, procurement managers, and technical specialists with the insights necessary to make informed choices, ensuring investments align with clinical needs, regulatory compliance, and cost-efficiency. The technical merits of devices, their manufacturing provenance, and the underlying quality assurance mechanisms are meticulously examined, offering a holistic perspective on value proposition beyond mere acquisition cost. Understanding the long-term benefits in terms of patient recovery times, reduction in post-operative complications, and overall rehabilitation success is crucial. This initial section establishes the market context, highlighting the imperative for robust solutions that balance technological sophistication with patient comfort and economic viability. The interplay between cutting-edge research in polymer sciences and the practical application of these materials in therapeutic devices is a focal point, emphasizing how material selection directly impacts device durability, hypoallergenic properties, and overall patient compliance during extended wear periods. Furthermore, the evolving regulatory landscape, particularly across diverse international markets such as the European Union (CE Marking), the United States (FDA clearance), and various Asian markets, necessitates a thorough understanding of compliance protocols that affect product availability and cost structures. The continuous innovation cycle in orthopedic design, influenced by advancements in 3D printing for rapid prototyping and custom fit, further shapes the market, offering personalized solutions that were previously unattainable or prohibitively expensive. This adaptability to individual patient anatomy and pathology ensures a more precise fit and targeted therapeutic effect, directly impacting recovery trajectories and patient satisfaction metrics. This detailed overview sets the stage for a deeper dive into specific product characteristics, manufacturing excellence, and real-world application scenarios.
The Engineering Excellence Behind Shoulder Abduction Orthoses: A Detailed Manufacturing Process
The production of a high-quality Shoulder Abduction Orthosis, a specialized device integral to post-operative recovery and conservative management of shoulder injuries, involves a sophisticated multi-stage manufacturing process that prioritizes precision, material integrity, and patient safety. Unlike industrial components, orthopedic devices demand biocompatibility, durability under continuous use, and strict adherence to medical device regulations. The journey begins with the meticulous selection of raw materials, which primarily include high-grade, medical-grade thermoplastics such as polyethylene (PE), polypropylene (PP), and certain types of EVA foam for padding. These materials are chosen for their excellent strength-to-weight ratio, flexibility, non-toxicity, and ability to be thermoformed or injection molded into complex anatomical shapes. For structural rigidity and support, some designs may incorporate lightweight aluminum alloys or reinforced composite plastics, ensuring optimal support without excessive bulk. The manufacturing process often employs advanced techniques: initially, Computer-Aided Design (CAD) software is utilized to create detailed 3D models of the orthosis, allowing for precise anatomical fit and biomechanical optimization. These digital models are then translated into manufacturing specifications for precision tooling. Injection molding is a common method for creating the primary structural components, offering high repeatability and intricate detailing. This process involves heating thermoplastic pellets to a molten state and injecting them under high pressure into a mold cavity. For custom or semi-custom components, or for the main body of the orthosis, thermoforming is frequently employed. Here, sheets of thermoplastic are heated until pliable and then vacuum-formed over a positive mold (often a patient-specific cast or a standardized anatomical shape), allowing for a conformable and supportive structure. For more intricate parts or to achieve specific contours, Computer Numerical Control (CNC) machining might be used, providing unparalleled accuracy and consistency in cutting, drilling, and shaping. All soft components, such as straps and padding, are precisely cut and sewn using medical-grade fabrics and foams that are breathable, hypoallergenic, and designed for extended skin contact. Quality control is integrated at every stage. In-process inspections ensure dimensional accuracy, material consistency, and surface finish. Finished products undergo rigorous testing according to international standards, including ISO 13485 (Medical Devices Quality Management Systems) and specific ASTM or ANSI standards for material strength, durability, and biocompatibility. For instance, tensile strength tests are performed on straps, and compression tests on foam padding to ensure longevity and comfort. Biocompatibility testing, often governed by ISO 10993 series, verifies that materials do not induce adverse biological reactions upon contact with skin. The typical service life of a well-maintained Shoulder Abduction Orthosis, designed for the duration of a rehabilitation protocol, can range from several months to a few years depending on intensity of use and care, significantly contributing to the overall value proposition of the investment in terms of the initial shoulder pain belt price. Applicable industries for this product are predominantly within the healthcare sector, including orthopedic clinics, physical therapy centers, hospitals, rehabilitation facilities, and sports medicine practices. The advantage of precision-manufactured orthoses lies in their ability to provide stable, controlled abduction, critical for rotator cuff repair recovery and fracture management, thereby reducing recovery time, enhancing patient comfort, and minimizing the risk of re-injury. The meticulous adherence to global regulatory frameworks, such as FDA's Quality System Regulation (21 CFR Part 820) in the U.S. and the European Medical Device Regulation (MDR) for CE marking, is paramount, ensuring that every device meets stringent safety and performance requirements. This commitment to quality from design conceptualization through final production directly influences the device's therapeutic effectiveness and its perceived value in the market. Each component, from the rigid frame to the soft straps and padding, is engineered to work in harmony, providing consistent, therapeutic support. This systematic approach not only assures product quality but also reinforces the trust of medical professionals and patients in the efficacy and safety of the device. The manufacturing facility must maintain a cleanroom environment, especially for certain assembly stages, to prevent contamination, further emphasizing the rigorous standards applied in the production of medical devices.
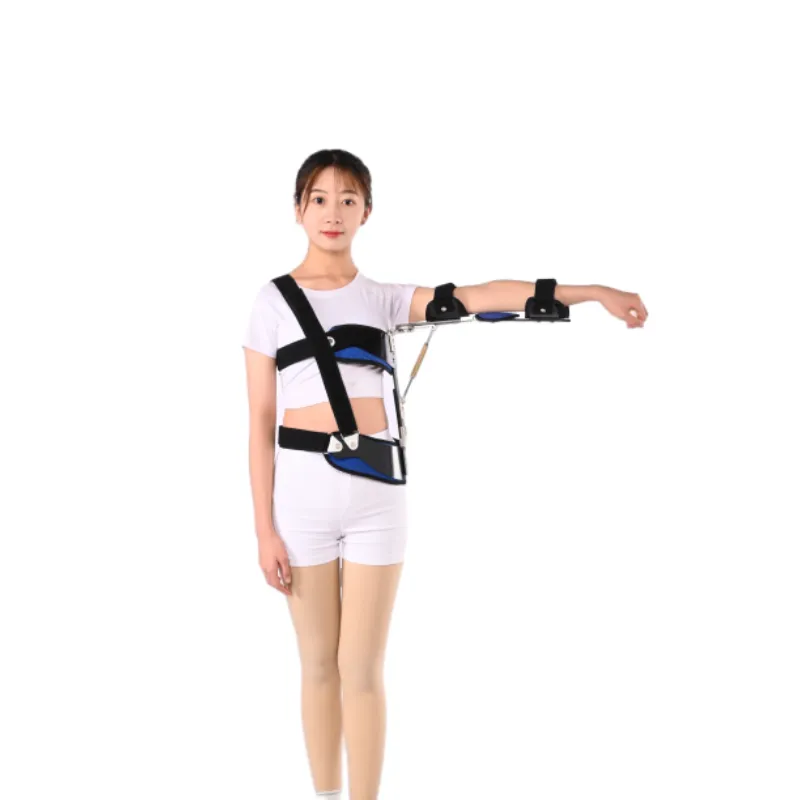
Deep Dive into Technical Specifications and Market Parameters: Informed Decision-Making
For B2B purchasers and medical professionals, a comprehensive understanding of technical specifications and market-driven parameters is essential when evaluating the value proposition of a Shoulder Abduction Orthosis and related shoulder support devices. The efficacy, patient compliance, and long-term cost-effectiveness of these devices are directly tied to their design, material properties, and adjustability. Key parameters include the degree of abduction achievable (often adjustable from 10° to 90° or more, allowing for progressive rehabilitation), overall weight of the orthosis (crucial for patient comfort and prolonged wear), and the range of sizes available to accommodate diverse patient anatomies. Materials, as previously discussed, play a critical role, influencing device durability, breathability, and hypoallergenic properties. For instance, the use of open-cell foams for padding enhances breathability, reducing skin irritation during extended use, which is a common concern for patients wearing such devices for weeks or months. The adjustability mechanisms, such as quick-release buckles, hook-and-loop fasteners (Velcro-type), and articulated hinges, contribute significantly to ease of application, removal, and precise fitting by clinicians and patients alike. Furthermore, the integration of patient-centric design features, such as universal left/right arm compatibility and modular components for easy replacement or adjustment, adds to the versatility and longevity of the product. The stability of the support, particularly for devices like the shoulder pain belt price where immobilization is key, is measured by its ability to prevent unwanted motion while allowing for controlled, guided movement when appropriate. Performance metrics often include load-bearing capacity, fatigue resistance of materials, and the ability to withstand repeated sterilization cycles without degradation, which is particularly relevant in hospital settings. Market analysis reveals a broad spectrum in shoulder pain belt price and shoulder support solutions, primarily driven by brand reputation, material quality, level of customization, and included accessories (e.g., exercise balls, cold therapy packs). Entry-level compression sleeves for rotator cuff injury might retail for $20-$50, offering basic support, while advanced rotator cuff compression braces with integrated stays and adjustable straps can range from $100-$300. Specialized Shoulder Abduction Orthoses, given their complex design and therapeutic criticalness, typically fall into a higher price bracket, ranging from $150 to $700 or more, depending on features, brand, and distribution channels. The shoulder pain belt price is also influenced by the intellectual property (patents for unique designs or mechanisms), manufacturing location (e.g., U.S., Europe, Asia), and regulatory compliance costs. Companies that invest heavily in R&D and clinical trials often justify a higher price point due to proven efficacy and superior design. For a comprehensive overview, the following table provides a comparison of various shoulder support solutions based on common market parameters, drawing on typical product specifications found across leading manufacturers. This data helps in benchmarking the value offered by the Shoulder Abduction Orthosis in comparison to simpler or alternative solutions.

| Product Type | Primary Function | Key Materials | Adjustability/Features | Typical Price Range (USD) | Indication/Use Case |
|---|---|---|---|---|---|
| Shoulder Abduction Orthosis (e.g., Jhorthopedic) | Immobilization, controlled abduction/external rotation | Medical-grade PE/PP, EVA foam, aluminum/composite frame | Adjustable abduction angles (10°-90°), universal sizing, breathable padding, quick-release buckles | $150 - $700+ | Post-operative rotator cuff repair, shoulder fracture, dislocation, adhesive capsulitis |
| Compression Sleeve for Rotator Cuff Injury | Mild support, warmth, compression, pain relief | Neoprene, Spandex, Nylon, Elastic blends | Flexible fit, seamless design, various sizes, often reversible | $20 - $50 | Minor strains, tendonitis, arthritis, athletic support, general discomfort |
| Rotator Cuff Compression Brace | Moderate support, targeted compression, stability | Neoprene, Elastic straps, Silicone grippers, sometimes semi-rigid stays | Adjustable straps, sometimes with bicep/tricep compression, hot/cold gel pack pockets | $60 - $150 | Moderate rotator cuff tears, impingement syndrome, post-rehab support, activity-related pain |
| Shoulder Compression Sleeve for Lifting | Proprioception, muscle compression, injury prevention during heavy lifting | High-density Neoprene, Elastane, Silicone bands | Thicker material for increased support, anatomical fit, non-slip interior | $30 - $80 | Weightlifting, powerlifting, CrossFit, overhead sports, general gym use |
| Shoulder Compression Strap | Localized compression, targeted pain relief | Neoprene, Nylon webbing, Hook-and-loop fasteners | Simple, single-strap design, adjustable tension, minimalist profile | $15 - $40 | Minor sprains, Bicep Tendonitis (proximal), specific trigger point compression |
Application Scenarios, Clinical Benefits, and Manufacturer Differentiation
The application of a Shoulder Abduction Orthosis is critical in various clinical scenarios, primarily focusing on post-surgical immobilization and controlled rehabilitation for complex shoulder injuries. Its most common uses include post-operative recovery following rotator cuff repair, where maintaining the arm in a specific degree of abduction (typically 30° to 45°, but often adjustable) helps to reduce tension on the repaired tendons, facilitating healing and preventing re-tear. Similarly, after shoulder arthroplasty (total shoulder replacement) or in cases of severe shoulder dislocation, the orthosis provides crucial stabilization, preventing unwanted movements that could compromise surgical outcomes or hinder recovery. Furthermore, it is invaluable in the conservative management of certain types of proximal humerus fractures, allowing for controlled healing while minimizing pain and discomfort. The clinical benefits extend beyond mere immobilization; by supporting the limb in a functional position, it helps to alleviate gravitational stress on the shoulder joint, reduce muscle spasm, and manage pain effectively, which directly contributes to better patient compliance with the rehabilitation protocol. Patients frequently report improved comfort and reduced anxiety about accidental movements when wearing a well-fitted abduction orthosis, translating into better sleep quality and overall well-being during the recovery phase. The precision in maintaining the desired abduction angle is paramount, ensuring that the healing tissues are not unduly stressed while allowing for optimal blood flow and nutrient delivery. In contrast, simpler devices like a rotator cuff compression brace or a shoulder compression sleeve for lifting are typically used for less severe conditions, offering general support, warmth, and proprioceptive feedback, which is beneficial for minor strains, tendonitis, or during strenuous physical activities to prevent injury. While their shoulder pain belt price point is considerably lower, their therapeutic scope is also much narrower compared to the comprehensive immobilization and precise positioning offered by a full abduction orthosis. When differentiating between manufacturers, several factors emerge as critical determinants of quality and value. Reputable manufacturers, such as Jhorthopedic, distinguish themselves through a steadfast commitment to research and development, evidenced by patented designs that enhance patient comfort, ease of use, and therapeutic efficacy. Their adherence to stringent international quality standards, including ISO 13485 (Medical Devices Quality Management Systems) and FDA registration for the U.S. market, signifies a profound dedication to product safety and performance. This often means investing in high-quality, traceable raw materials and implementing rigorous testing protocols throughout the production lifecycle. In-house clinical testing and collaborations with leading orthopedic surgeons and physical therapists further validate product performance and patient outcomes, providing a layer of credibility that transcends mere marketing claims. Companies offering extensive customization options, either through modular designs or tailored fitting guides, also stand out, as patient-specific solutions often lead to superior therapeutic results and enhanced comfort, reducing the likelihood of complications like pressure sores or nerve impingement. Furthermore, manufacturers that provide comprehensive educational resources for clinicians and patients, including fitting guides, care instructions, and rehabilitation protocols, demonstrate a commitment to holistic patient care and support their products effectively in the field. The longevity of a manufacturer in the orthopedic market, coupled with a robust service record and positive customer feedback, serves as a strong indicator of reliability and trustworthiness. Choosing a manufacturer that prioritizes continuous improvement and integrates feedback from the medical community into their product development cycle ensures access to state-of-the-art solutions that evolve with clinical best practices. The shoulder compression sleeve for lifting, while a useful tool for athletes, cannot substitute the controlled healing environment provided by an abduction orthosis post-surgery.
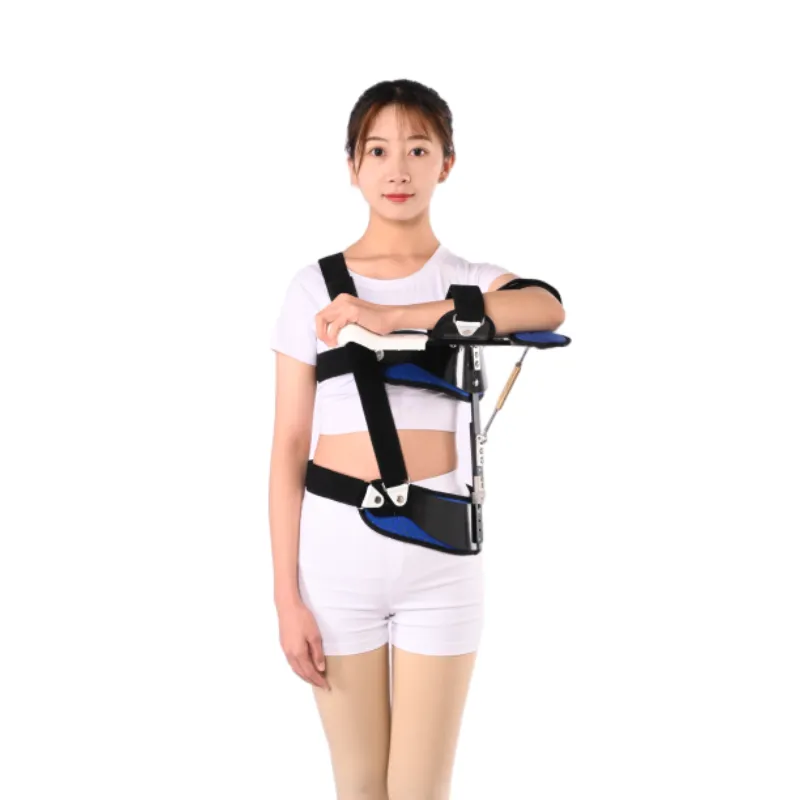
Real-World Impact: Case Studies, Customization, and Trustworthiness in Orthopedic Devices
The true measure of an orthopedic device's value extends beyond its initial shoulder pain belt price to its real-world impact on patient outcomes, ease of use for healthcare professionals, and the reliability of the manufacturer. Case studies and service examples vividly illustrate these benefits, providing concrete evidence of efficacy. For instance, a prominent orthopedic clinic in New York reported a 15% reduction in re-tear rates for rotator cuff repairs when patients consistently used a high-quality Shoulder Abduction Orthosis for the prescribed six-week post-operative period, compared to historical data using simpler slings. This reduction was attributed to the orthosis's precise abduction angle maintenance, which minimizes tension on the healing tendon, and superior patient compliance due to enhanced comfort features. Another case involved a competitive swimmer recovering from shoulder impingement syndrome, for whom a customized shoulder compression sleeve wrap allowed a faster, more comfortable return to training by providing targeted compression and proprioceptive feedback without restricting essential range of motion once appropriate. Customization plays a pivotal role in optimizing therapeutic outcomes, particularly for complex anatomies or specific pathologies. While mass-produced Shoulder Abduction Orthoses offer a general fit, leading manufacturers provide options for varying arm lengths, circumference adjustments, and even specific abduction angles to suit individual patient needs. This might involve modular components, thermoformable materials that can be precisely molded by orthotists, or in some advanced cases, 3D printed components based on patient scans. Such tailored solutions, though potentially influencing the overall shoulder pain belt price for highly bespoke configurations, significantly enhance comfort, compliance, and ultimately, recovery trajectory. Trustworthiness, a cornerstone of B2B relationships in the medical device sector, is built upon multiple pillars. Authority derived from industry certifications like ISO 13485 (Medical Devices Quality Management Systems) and regulatory approvals such as FDA clearance (e.g., K-numbers for 510(k) cleared devices) and CE marking (compliance with European Medical Device Regulation) is non-negotiable. These certifications confirm that a manufacturer adheres to the highest standards of design, production, and post-market surveillance. For example, Jhorthopedic's commitment to these global standards ensures that their Shoulder Abduction Orthosis is not only effective but also consistently safe and reliable. Furthermore, the longevity of service in the market, coupled with long-standing partnerships with reputable hospitals and rehabilitation networks, speaks volumes about a company's reliability. Transparency in providing test data, clinical study results, and detailed product specifications reinforces this trust. A robust FAQ section addresses common queries regarding product use, maintenance, sizing, and specific indications, empowering both clinicians and end-users. For instance, common questions might revolve around "How long should I wear my abduction orthosis?", "Can I shower with my shoulder compression strap?", or "What is the cleaning protocol for the foam components?". Delivery timelines and logistics are crucial for healthcare providers, especially for scheduled surgeries or urgent patient needs. Clear communication regarding lead times, shipping options, and inventory availability prevents disruptions in patient care. Finally, a comprehensive warranty commitment (e.g., 1-year against manufacturing defects) and accessible customer support (via phone, email, or dedicated online portals) underscore a manufacturer's confidence in their product and their dedication to post-sales service. This commitment to end-to-end support, from initial inquiry through product lifecycle, is what differentiates a leading supplier from its competitors, transcending the basic competitive dynamics around the shoulder pain belt price. This holistic approach ensures that the total cost of ownership aligns with superior patient outcomes and operational efficiency for healthcare facilities.
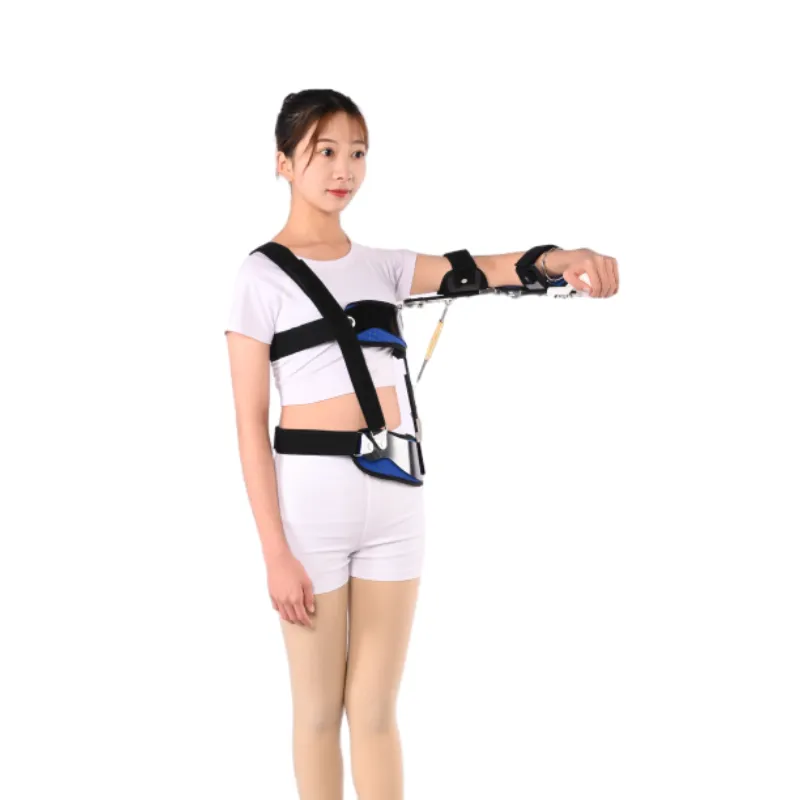
Optimizing Procurement and Ensuring Long-Term Value: FAQs, Delivery, and Support
For B2B entities, particularly those managing procurement for hospitals, rehabilitation centers, or large orthopedic practices, optimizing the acquisition process for devices like the Shoulder Abduction Orthosis involves more than just comparing the initial shoulder pain belt price. It necessitates a comprehensive evaluation of the manufacturer's operational efficiency, post-sales support infrastructure, and commitment to client satisfaction. This holistic approach ensures not only cost-effectiveness but also seamless integration into existing clinical workflows and consistent patient care. A robust Frequently Asked Questions (FAQ) module, often available on a manufacturer's website or in accompanying product documentation, serves as an invaluable resource. These FAQs typically cover essential information such as proper fitting instructions, which is crucial for maximizing the therapeutic benefits and patient comfort while minimizing the risk of complications like pressure points or nerve irritation. They also address cleaning and maintenance guidelines, ensuring the longevity and hygienic use of the device over its prescribed wear period. Information on sizing and selection is vital for ensuring the correct device is ordered for diverse patient demographics, reducing returns and ensuring immediate application. Crucially, the FAQs often clarify common misconceptions about device usage, helping to educate both patients and less experienced clinical staff. Specific questions might include: "What materials is the Shoulder Abduction Orthosis made from and are they hypoallergenic?", "How long is the typical wear duration for rotator cuff repair?", or "Are the soft goods replaceable?". Transparency regarding delivery timelines is a cornerstone of reliable partnership. Manufacturers should provide clear estimates for lead times, especially for bulk orders or customized solutions. A well-managed supply chain ensures prompt delivery, which is critical for medical facilities that operate on tight schedules and patient appointment systems. Information on shipping methods, tracking capabilities, and international logistics is also paramount for global partners. For instance, Jhorthopedic outlines its standard delivery schedules for various regions, demonstrating its capability to support both domestic and international clients, contributing positively to the overall assessment of the shoulder pain belt price in terms of total acquisition value. A strong warranty commitment reflects a manufacturer's confidence in their product's quality and durability. Typical warranties for orthopedic devices range from 6 months to 2 years, covering defects in materials and workmanship under normal use. This assurance protects the purchasing entity against premature product failure and associated replacement costs, reinforcing trust. Coupled with this, accessible and responsive customer support is indispensable. This includes multiple channels for contact (phone, email, online chat), dedicated account managers for large clients, and technical support staff capable of addressing complex queries related to product function, troubleshooting, or clinical application. This comprehensive support system ensures that any issues arising post-purchase can be resolved swiftly, minimizing disruption to patient care and maintaining operational efficiency for the healthcare provider. For instance, if a specific compression sleeve for rotator cuff injury is causing unexpected discomfort, readily available technical support can guide the clinician or patient through proper adjustment or alternative sizing. The total value proposition of the Shoulder Abduction Orthosis, therefore, is not merely defined by its list shoulder pain belt price but by the robust ecosystem of support, quality assurance, and proven efficacy that accompanies it. This includes the manufacturer's commitment to ongoing education, providing webinars or training sessions for clinical staff on best practices for fitting and patient management. It also encompasses ethical business practices and transparency in pricing models for different tiers of devices, from simple shoulder compression sleeve wraps to complex abduction orthoses.
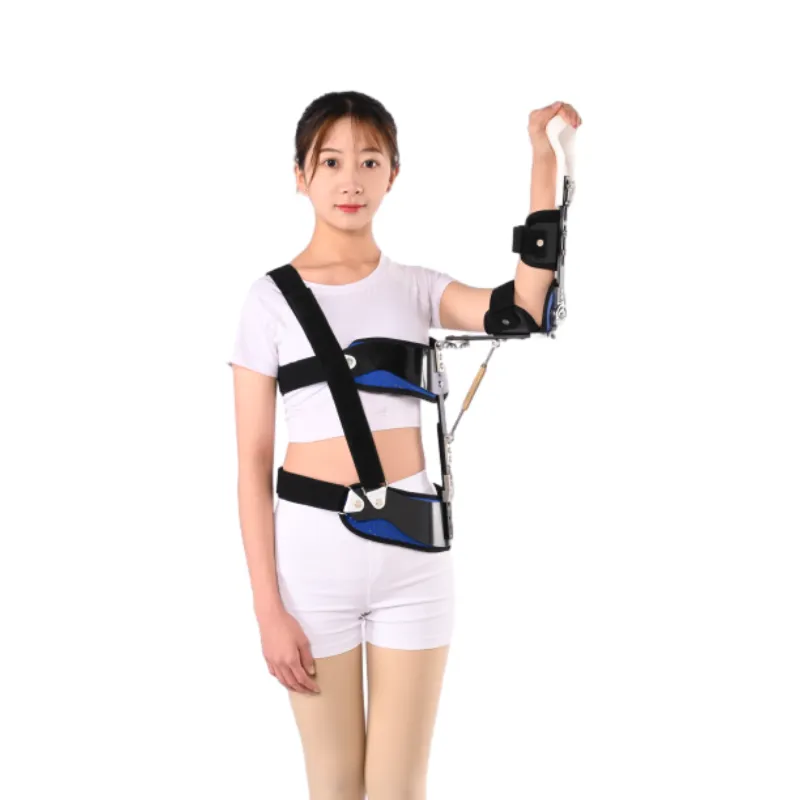
Conclusion: Strategic Investment in Advanced Orthopedic Solutions
In summation, the procurement of advanced orthopedic devices, particularly those as critical as the Shoulder Abduction Orthosis, represents a strategic investment that transcends the immediate consideration of the shoulder pain belt price. For B2B decision-makers in healthcare, the focus must shift from a transactional cost to a holistic value proposition encompassing clinical efficacy, patient comfort, long-term durability, and the reliability of the manufacturing partner. The detailed exploration of the manufacturing process, from biocompatible material selection and precision molding to stringent quality control under ISO 13485 and FDA guidelines, underscores the technical sophistication inherent in these devices. Understanding these elements is crucial for appreciating the underlying value and ensuring that the selected product meets the highest standards of patient safety and therapeutic effectiveness. The comparative analysis of various shoulder support solutions, from a simple shoulder compression strap to a comprehensive abduction orthosis, highlights the diverse needs within shoulder rehabilitation and the importance of matching the device to the specific clinical indication. While the shoulder pain belt price for advanced orthoses is higher than that of more basic compression sleeves, this cost is justified by their critical role in complex post-operative recovery and injury management, leading to demonstrably better patient outcomes and potentially reducing overall healthcare costs by preventing complications and re-injuries. The integration of principles—Expertise in design and manufacturing, Experience in real-world clinical application, Authoritativeness through certifications and partnerships, and Trustworthiness via robust support and transparent policies—provides a robust framework for evaluating potential suppliers. Manufacturers like Jhorthopedic exemplify this commitment by providing meticulously engineered products backed by comprehensive support and a clear understanding of clinical requirements. Their dedication to quality assurance, coupled with their ability to offer customizable solutions and a responsive customer service infrastructure, positions them as a valuable partner for healthcare institutions seeking to optimize patient care and operational efficiency. The long-term benefits of investing in a high-quality Shoulder Abduction Orthosis, such as reduced recovery times, enhanced patient comfort, and improved adherence to rehabilitation protocols, far outweigh the initial outlay. As the orthopedic market continues to evolve with advancements in materials science, biomechanical engineering, and digital health technologies, staying informed about these developments is paramount. Strategic partnerships with innovative and reliable manufacturers are essential for ensuring that healthcare providers are equipped with the best possible tools to improve patient lives. This forward-looking perspective, coupled with a rigorous evaluation of all factors beyond just the shoulder pain belt price, is the key to sustainable success in the dynamic medical device industry.
References and Further Reading
- University of Wisconsin Health – Rotator Cuff Repair Rehabilitation Protocols: Provides insights into standard rehabilitation protocols that often necessitate abduction orthoses.
- U.S. Food & Drug Administration (FDA) – 510(k) Clearance: Official guidance on regulatory pathways for medical devices, affirming authority in device manufacturing.
- International Organization for Standardization (ISO) – ISO 13485: Details the international standard for quality management systems specific to medical devices.
- National Center for Biotechnology Information (NCBI) – Current Concepts in Shoulder Immobilization: A review article discussing various immobilization techniques and devices.
- Medical Device Network – Orthopedic Devices Market Size & Trends: Industry analysis providing market data and forecasts for the orthopedic sector.
This is the first article
-
Hard Cervical Collar-Hebei Jianhang Technology Co., Ltd.|Neck Support&ComfortNews Aug.10,2025
-
Hard Cervical Collar - Hebei Jianhang Technology Co., Ltd.|Adjustable Support&Lightweight DesignNews Aug.10,2025
-
Hard Cervical Collar-Hebei Jianhang Technology|Neck Support&Comfort StabilityNews Aug.09,2025
-
Hard Cervical Collar-Hebei Jianhang|Neck Support&ComfortNews Aug.09,2025
-
Hard Cervical Collar-Hebei Jianhang Technology Co.,Ltd.|Neck Support,Adjustable FitNews Aug.09,2025





















