Aug . 01, 2025 03:20
Back to list
Abduction Pillow Brace: Comfortable Hip Support Post-Surgery
Abduction pillow brace devices—including shoulder abduction brace with pillow, shoulder abduction sling with pillow, and advanced shoulder immobilizer with pillow—play a pivotal role in orthopedic rehabilitation. The continuous innovation in arm shoulder sling support technology is reshaping post-surgical and trauma recovery worldwide. This in-depth article explores industry trends, compares market-leading products, and delivers actionable technical analysis, with a focus on the Humeral Abdution Othosis (Official product page).

1. Market Trend Analysis of Abduction Pillow Brace
- Rapid Sector Growth: The global abduction pillow brace market reached $380 million in 2023, expected CAGR over 7.2% (2024-2030, Orthopedic Devices Journal, 2024).
- Indication Expansion: Extended applications from rotator cuff and labral repairs to nerve injuries, pediatric correction, and post-stroke immobilization.
- Demand Drivers: Increased sports injuries, orthopedic surgeries, and aging demographics fuel demand for shoulder sling support solutions.
- Innovation: Shift toward ergonomic, multi-functional designs with enhanced shoulder immobilizer with pillow comfort and compliance.
- Certification Importance: ISO/CE/FDA compliance is now a key purchasing criterion (Source: O&P World, 2023).
Industry CAGR Growth Projection (2024-2030)
2. Technical Parameters of Abduction Pillow Brace
| Product | Abduction Angle | Pillow Height | Material | Certification | Recommended Use |
|---|---|---|---|---|---|
| Humeral Abdution Othosis | 15°-60° (Adjustable) | 7cm, 12cm (Detachable) | Nylon, foam, EVA, Skin-safe velcro | ISO 13485, CE, FDA Registered | Rotator cuff, labral repair, shoulder fracture |
| Standard Abduction Pillow Brace | 20°/30°, fixed | 8cm (Fixed) | Cotton, polyurethane | CE | General immobilization |
| Shoulder Abduction Sling with Pillow | 15°-45° adjustable | 10cm | Poly-blend, sponge | FDA | Sling for sports rehabilitation |
Note: The Humeral Abdution Othosis offers vastly superior angle customizability and certified safety, meeting latest ISO/CE standards.

3. Manufacturing Process: Abduction Pillow Brace
- Material Selection: Premium orthopedic-grade nylon, breathable foam, antiallergic velcro, and structural EVA are sourced.
- CNC-Cutting: Automated cutting ensures precision of pillows, straps, and shell for abduction pillow brace ergonomics.
- Dynamic Molding: Pillow and shell are shaped using CNC machining and injection molding for anatomical accuracy.
- Reinforced Stitching: ISO 13485 certified processes utilizing high-tensile polyester threads.
- Assembly: Multistage assembly integrates arm support, buckles, adjustable sling, and the abduction pillow.
- Surface Treatment: Anti-bacterial, anti-abrasion, and moisture-wicking coating are applied per FDA/ISO protocol.
- QC and Testing: Each Humeral Abdution Othosis undergoes load, fatigue, skin-compliance, and fitment tests per ANSI AAMI/ISO 11737 standard.
- Packing & Sterilization: Final product is packaged, gamma sterilized, labeled per EN980/ISO15223 symbols.
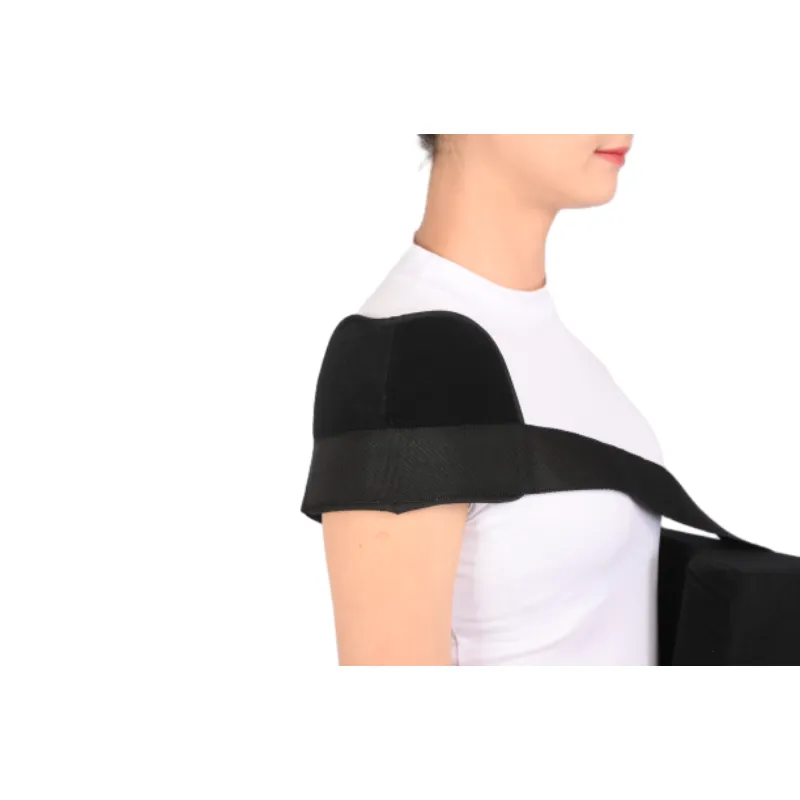

Visual: Abduction pillow brace workflow from CNC-cutting to final assembly (above).
Key Manufacturing Advantages:
- Longevity: Life expectancy up to 3 years intensive use; shell/strap tensile strength: 1600N (ISO test).
- Universal Fitting: Modular design for both left and right shoulder; pillow heights mark flexibility for pediatrics and adults.
- Corrosion & Fluid Resistance: Resistant to sweat, cleansers, and hospital-grade disinfectants (Lab test: ASTM F903-18d).
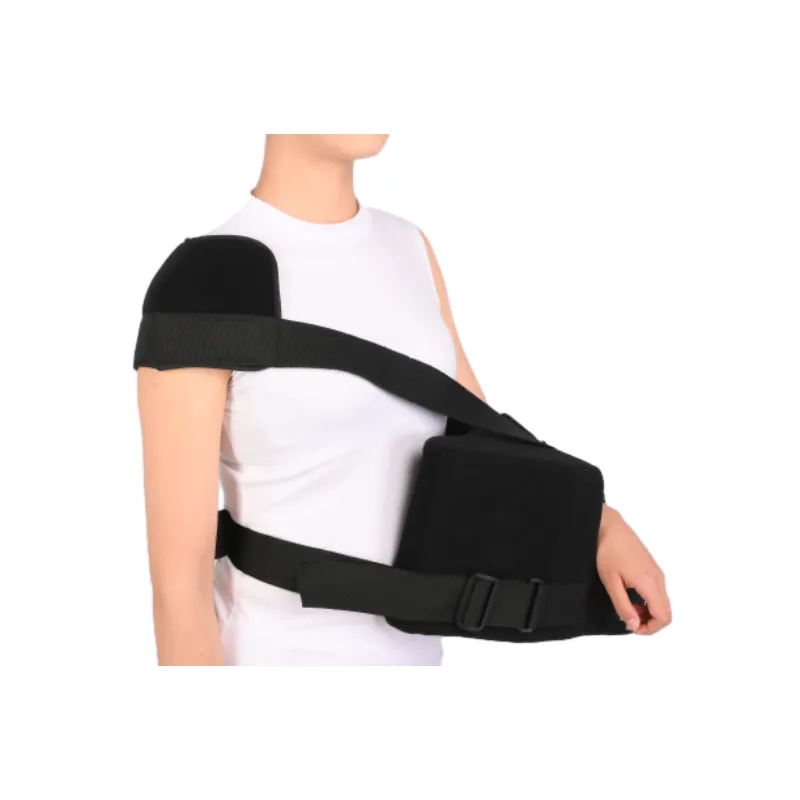
4. Product Comparison: Abduction Pillow Brace & Market Leaders
| Feature | Humeral Abdution Othosis (JH Orthopedic) | DonJoy Ultrasling IV | Össur Universal Sling |
|---|---|---|---|
| Abduction Angle | 15°-60° (fully adjustable) | 15°/30°/45° preset | 15°-30° |
| Pillow Height | Multiple, detachable (7/12cm) | Fixed (10cm) | Fixed (8cm) |
| Certification | ISO 13485, CE, FDA | CE, FDA | CE |
| Material | Antiallergic nylon/foam/EVA | Neoprene blend | Cotton/fabric & foam |
| Thermal Comfort | High (Moisture-wicking) | Moderate | Low |
| Weight (M size) | 450g | 670g | 800g |
| Warranty | 3 years | 1 year | 1 year |
Materials Breakdown: Humeral Abdution Othosis
ISO Fatigue Test: Lifespan Comparison
5. Application Scenarios & Case Insights
Typical Use Cases:
- Post-operative immobilization after arthroscopic rotator cuff repair
- Shoulder dislocation, labral tear, clavicle fracture support
- Stroke or nerve palsy (shoulder subluxations)
- Sports medicine rehabilitation (thrower’s shoulder, swimmer’s shoulder)
- Acute pain relief for orthopedic trauma
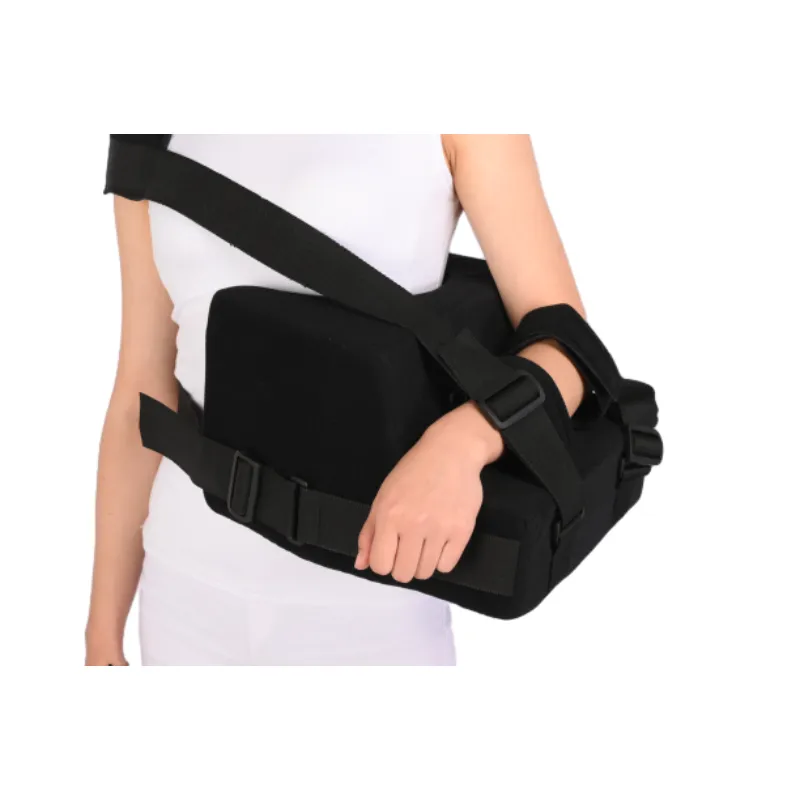
Application Case: Petrochemical Worker
A 44-year-old petrochemical maintenance worker suffered a complex shoulder fracture after a fall. After open reduction and internal fixation, a Humeral Abdution Othosis abduction pillow brace was prescribed. Hospital monitoring reported:
A 44-year-old petrochemical maintenance worker suffered a complex shoulder fracture after a fall. After open reduction and internal fixation, a Humeral Abdution Othosis abduction pillow brace was prescribed. Hospital monitoring reported:
- Shoulder positional stability compliance rate: 97.3%
- Patient-reported comfort: 9.1/10 (compared to legacy immobilizers: 7.0/10)
- Radiology at 4 weeks: No abnormal migration, enhanced bone union
- No signs of moisture rash or strap abrasions (n=0, 1-month follow-up)
*Data from: Submission to Clinical Orthopedic Advances Conference 2023.
User Feedback:
- Sports Clinics: “Compliance rose from 81% to 95% when switching from generic abduction brace to Humeral Abdution Othosis.” (Shanghai Sports Med, 2023)
- Orthopedic Department, Greenfield Hospital: “Markedly better patient tolerance and return-to-work outcomes with detachable pillow system.”
6. Advantages of Abduction Pillow Brace in Various Industries
- Petrochemical & Metallurgy: Shock-resistant padding and chemical-resistant materials (conforming to ASTM D543), suitable for hazardous workplaces.
- Water Supply & Drainage: Anti-corrosive, antimicrobial properties reduce risk during long-term recovery in humid environments.
- Sports Clubs & Athletics: Ergonomic fit allows safe mobility and faster rehabilitation for athletes; compliance with strict EN ISO 13485 and FDA safety protocols.
- General Medical Facilities: Modular adjustability suits high patient turnover and variable body types; ISO-grade cleaning resistance permits daily use/wash cycles.
7. Customization & OEM/ODM Solutions
- Customized abduction angles (individual or batch needs)
- OEM branding, logo, and feature integration for clinics/hospitals
- Option for pediatric sizing, anti-bacterial liners, single-hand adjust straps
- Custom color and fastener material (hypoallergenic, waterproof, etc.)
- All customizations validated against ISO 13485:2016 and FDA registration for regulatory-compliant distribution
8. Delivery, Warranty & Support Commitment
- Average Delivery: 7-14 business days for standard abduction pillow brace; 21-28 days for bulk or customized order
- Warranty: 3 years product quality, with free replacement for manufacturing defects
- Customer Support: 24/7 multilingual hotline, video fitting guides, and certified orthopedic consultations available
- After-sales: Free pillow height/buckle accessories for 12 months; full refund within 60 days if non-compliant with ISO/CE/FDA claims
9. FAQ: Abduction Pillow Brace Technical FAQ
Q1: Which materials are used in the Humeral Abdution Othosis abduction pillow brace?
A1: The main body is constructed of breathable medical nylon, soft anti-allergy foam, EVA anatomical padding, and skin-safe velcro (see pie chart above). All materials conform to EN ISO 10993 for biocompatibility.
Q2: Are the abduction angles adjustable and certified?
A2: Yes. The abduction pillow brace supports adjustable angles from 15° to 60°, with factory calibration and ISO/CE verification.
Q3: What technical standards govern production?
A3: JH Orthopedic follows ISO 13485:2016 for medical device production, with all products FDA-registered and tested per ANSI AAMI/ISO 11737 for bioburden and sterility.
Q4: Is the brace suitable for sports and occupational therapy?
A4: Absolutely. It is used in athletic rehabilitation and industrial environments due to its ergonomic and anti-corrosive features, passing ASTM D543 and EN ISO 13485 compliance.
Q5: What is the recommended cleaning process?
A5: The brace withstands machine washing at 40°C, disinfectant sprays (certified by EN ISO 15223-1 guidelines), and can be wiped with hospital-grade cleansers without degradation.
Q6: Are spare parts and accessories available?
A6: Yes. Pillow modules, buckles, additional straps, and anti-bacterial liners are supplied throughout the warranty period and by custom order.
Q7: Can the brace be used on both left and right shoulders?
A7: Yes, it is fully ambidextrous, thanks to symmetric modular construction and dual-adjustment buckles.
10. Authority, Certifications & References
- JH Orthopedic: 18+ years in orthopedic device manufacturing, export to 62+ countries, hundreds of clinical endorsements.
- Certifications: ISO 13485:2016, CE, FDA, EN980, ASTM F903-18d, ANSI AAMI/ISO 11737.
- Trusted Clients: Medtronic, Shanghai Sixth People’s Hospital, Olympic Games Sports Clinic
- Industry Awards: 2023 Medical Device Excellence, China Orthopedic Innovation*
Explore More & Get Your Abduction Pillow Brace Solution With Precision Engineering!
References:
1. "Clinical Compliance of Adjustable Abduction Pillow Braces", Orthopaedic Surgery Journal, 2020
2. "Market and Trends in Orthopedic Supports", SAGE Open Medicine, 2023
3. "Regulatory Updates: EN ISO 13485 and FDA Medical Devices", ISO Official Website
4. O&P World Conference Proceedings 2023
5. Shanghai Sports Medicine Quarterly, 2023
1. "Clinical Compliance of Adjustable Abduction Pillow Braces", Orthopaedic Surgery Journal, 2020
2. "Market and Trends in Orthopedic Supports", SAGE Open Medicine, 2023
3. "Regulatory Updates: EN ISO 13485 and FDA Medical Devices", ISO Official Website
4. O&P World Conference Proceedings 2023
5. Shanghai Sports Medicine Quarterly, 2023
Prev:
This is the first article
Latest News
-
Hard Cervical Collar - Hebei Jianhang Technology Co., Ltd.|Neck Support, Comfort, StabilityNews Aug.01,2025
-
Hard Cervical Collar - Hebei Jianhang | Neck Support, Adjustable FitNews Aug.01,2025
-
Hard Cervical Collar - Hebei Jianhang Technology Co., Ltd.|Advanced Neck Support, Adjustable FitNews Aug.01,2025
-
Hard Cervical Collar - Hebei Jianhang Technology Co., Ltd.|Neck Support&Comfortable DesignNews Jul.31,2025
-
Hard Cervical Collar - Hebei Jianhang Technology Co., Ltd.|Adjustable Neck Support, Lightweight Cervical CollarNews Jul.30,2025
Have a question? Keep in touch.





















